|
|
Guidelines
for Classification of
Medical Devices
|
Are you ready for Brexit impacts? Do you have a Brexit contingency plan?
You may need either an EU/EC European authorized representative based in EU-27 countries or a UK authorised representative based in UK, or may even need both EU & UK representatives, depending on different brexit scenarios.
Register/Notify your MD-Medical Devices & IVD-In Vitro Diagnostic Medical
Devices with MHRA in UK & other EEA (EU/EFTA) authorities by world-leading
consultancy- Wellkang team based in both UK (England) & EU-27 (Ireland).
Wellkang team can help you under all Brexit scenarios!
Click here to get FREE Guide Now! |
Depending on its Intended Purpose, a medical device may be classified as Class I (including Is & Im), Class IIa, IIb and III, with Class III covering the highest risk products. The higher the classification the greater the level of assessment required. All Class Is, Im, IIa, IIb and III medical devices require the intervention of third party: the so-called Notified Body.
Classification of a medical device will depend upon a series of factors, including:
- how long the device is intended to be in continuous use
- whether or not the device is invasive or surgically invasive,
- whether the device is implantable or active
- whether or not the device contains a substance, which in its own right is considered to be a medicinal substance and has action ancillary to that of the device.
The classification rules are set out in Annex IX of the directive. This annex includes definitions of the terminology used in the classification rules.
CONTENTS

It is not feasible economically nor justifiable in practice to subject all
medical devices to the most rigorous conformity assessment procedures
available. A graduated system of control is more appropriate. In such a
system, the level of control corresponds to the level of potential hazard
inherent in the type of device concerned. A medical device classification
system is therefore needed, in order to channel medical devices into the
proper conformity assessment route.
In order to ensure that conformity assessment under the Medical Device
Directive functions effectively from January 1995, manufacturers should
be able to know as early as possible in which class their product is.
Identification of the class of each individual type of device by a committee
procedure would have taken too long to achieve this goal. It was therefore
decided to set up a system of classification rules within the directive, so
that each manufacturer could classify its own devices.
A simple set of classification rules based on technical features of medical
devices existing now and in the future is impossible, because of the vast
number and the changing nature of variables involved. The human body,
however, is a relatively unchanging element of the equation. The
European legislator established therefore a classification concept which is
essentially based on potential hazards related to the use and possible
failure of devices taking account of technology used and of health policy
considerations. This approach in turn allows the use of a small set of
criteria that can be combined in various ways: duration of contact with the
body, degree of invasiveness and local vs. systemic effect.
It is recognized that although the existing rules will adequately classify
the vast majority of existing devices, a small number of difficult cases may
arise. Such cases may in particular include the determination of the
borderline between two classes. In addition there may be devices that
cannot be classified by the existing rules because of their unusual nature
or situations where the classification would result in the wrong level of
conformity assessment in light of the hazard represented by the device.
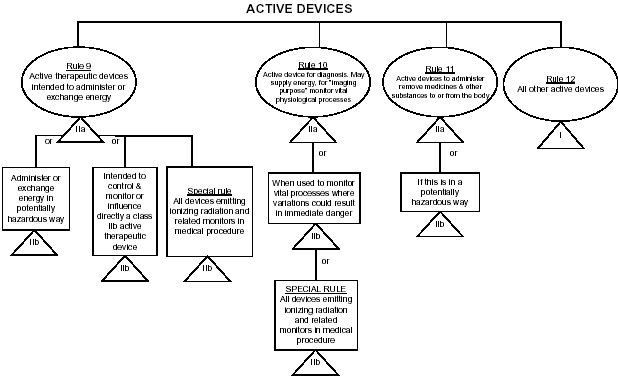
Graphical Summary
medical devices classification guidance chart
for initial identification of probable device class




 back to top of this page back to top of this page
Please click the following links to learn more about every CE-Marking-related European Union Directive, Guidelines to Directives, Frameworks of implementation of Directives, and Agreements on Mutual Recognition of conformity assessment between European Union and other countries such as USA, Japan, Canada, Australia, New Zealand and Israel:-
- What is CE marking (CE mark)?
- The CE marking logo (Free download CE mark logo)
- Download of the EC Rep symbol/logo
- CE Marking (CE mark) EU/EC Declaration of Conformity: definition and required content
- What is a manufacturer? What are the responsibilities of a manufacturer?
- Is the Own Brand Labeller or Private Labeller considered as the legal Manufacturer?
- Why is CE marking called "European passport"?
- Does my product need CE Marking in order to be sold/exported on/to the European Market?
- How to obtain CE Marking for my product?
- How can Wellkang Tech Consulting help me to obtain CE Marking for my product?
- Wellkang can be your Authorized Representative in Europe for CE Marking purpose !
- Complete list of all CE Marking Directives and Guidelines to Directives
- Complete list of all (more than 1000) Notified Bodies for CE marking
- Complete list of all European and/or international standards related to CE marking
- 85/374/eec: Directive of Liability for Defective Products (for all products)
- 1999/34/ec: Directive of Liability for Defective Products (amending) (for all products)
- 2000: (Proposal for a new) General Products Safety Directive (for all products)
- 92/59/eec: Directive of General Products Safety (for all products)
- 93/68/eec: "CE Marking" Directive (for all products)
- 93/465/eec: Conformity Assessment Procedures & CE Marking Rules, & Annex (for all products)
- Guide to Implementation of directives based on new approach & global approach (for all products)
- 73/23/eec: Directive of Low Voltage Electrical Equipment
- Guideline to Low Voltage Directive (LVD) 73/23/eec & Annex I, II
- 2000&2001 Framework of implementation of (LVD) Directive 73/23/eec
- 87/404/eec: Directive of Simple Pressure Vessels
- 88/378/eec: Directive of Toys & Annex I, II, ... IV
- 89/106/eec: Directive of Construction Products
- 89/336/eec: Directive of Electromagnetic Compatibility (EMC)
- Guideline to Directive of Electromagnetic Compatibility (EMC) 89/336/eec
- Communication 2000 in framework of implementation of EMC Directive 89/336/EEC
- 89/686/eec: Directive of Personal Protective Equipment (PPE)
- 90/384/eec: Directive of Non-automatic Weighing Instruments
- 90/396/eec: Directive of Appliances Burning Gaseous Fuels (AppliGas)
- 2001 Commission Communication in Framework of Impl (AppliGas) Directive 90/396/eec
- 92/42/eec: Directive of Efficiency of (Liquid or Gaseous fueled) Hot Water Boilers
- 93/15/eec: Directive of Explosives for Civil Uses
- 93/42/eec: Directive of Medical Devices
- 2001 Guidelines for Classification of Medical Devices
- Guidelines relating to the demarcation between Directives 90/385/eec, 93/42/eec and 65/65/eec.
- 90/385/eec: Directive of Active Implantable Medical Devices
- 98/79/ec: Directive of In Vitro Diagnostic Medical Devices
- Guidelines to Medical Devices Vigilance System
- List of Harmonised (European) Standards for Medical Devices
- List of Notified Bodies for Medical Devices Directive
- 93/65/eec: Directive of Air Traffic Management Equipment & Systems
- 94/9/ec: Directive of Equipment used in Potentially Explosive Atmospheres (Atex)
- Guidelines to directive 94/9/ec (Atex)
- 94/25/ec: Directive of Recreational Craft
- 95/16/ec: Directive of Lifts
- 96/48/ec: Directive of Trans-European High-speed Rail System
- 96/57/ec: Directive of Energy Efficiency: Household Refrigerators & Freezers
- 96/98/ec: Directive of Marine Equipment
- 98/37/ec: Directive of Machinery
- 2000: Comments on Directive of Machinery 98/37/ec
- 99/5/ec: Directive of Radio Equipment & Telecommunications Terminal Equipment (R&TTE)
- 2000/9/ec: Directive of Cableway Installations to Carry Persons
- 2000/14/ec: Directive of Noise Emission in the environment by equipment for use outdoors



| home/first page | back to where you were | back one page | top of this page | next page |

| |

